Imagine if identifying the right cancer treatment for a specific patient was more akin to a dating app where the best potential matches are suggested based on each person’s profile. While progress has been made in developing more targeted therapies for some cancers based on their genetic profile, this has been a challenge with sarcomas. These tumors, which can develop in bones or soft tissues (such as lymph nodes, blood vessels, nerves, fat, tendons, muscles, ligaments, or cartilage), have more than 100 subtypes with different genetic features. Not being able to classify these tumor subtypes can make it harder to match patients with the right treatment.
Currently, diagnostic errors for sarcomas have been found to range between 25% and 40%. This is in part due to the variety in tumor types, but also their rarity—sarcomas represent less than 1% of tumors in the United States (0.7% in soft tissue and 0.2% in bone) with only a few cases each year reported for some subtypes.
When it is possible to match a therapy to a specific genetic alteration found in sarcoma tumors, it can make a big difference. In a retrospective study published in Clinical Cancer Research, a journal of the American Association for Cancer Research (AACR), researchers found that patients with metastatic sarcomas in early-phase clinical trials for biomarker-matched therapies had an overall survival of 21.5 months compared to 12.3 months in patients treated with therapies not matched to their specific tumors. This supports the need to develop more targeted therapies for patients with metastatic sarcomas as they have a five-year survival rate of 17% compared to 83% in cases when the cancer has been caught before it spreads and can be removed via surgery.
Recent studies reveal some of the progress researchers are making in better profiling sarcoma tumors, developing targeted therapies, and in one case, monitoring and treating through a multi-layered solution.
Reclassifying Suspected Sarcoma Tumors
Currently, sarcoma tumors are classified mostly through techniques used to study morphology—in which a sample of the tumor is examined for different characteristics including its size, shape, and color—and immunohistochemistry (IHC) staining—in which antibodies are used to detect protein markers. Given the challenges associated with properly classifying sarcomas, researchers tested whether the use of whole-genome and whole-transcriptome sequencing (WGTS) could be a more effective option. This process captures information about both the DNA and RNA in cancer cells, including mutations, genetic signatures, and potential actionable targets.
In a prospective study of 200 patients with either a soft-tissue or bone tumor suspicious for malignancy, the use of WGTS resulted in reclassification of 7% of tumors, according to results published in Clinical Cancer Research, a journal of the AACR. This included four samples downgraded from low-grade sarcomas to benign lesions and two cases reclassified as metastatic malignant melanomas. Additionally, this process allowed researchers to identify treatment-relevant variants for 15% of malignant soft-tissue and bone tumors and 16% of high-grade sarcomas and metastatic lesions. In the case of gastrointestinal stromal tumors, WGTS identified relevant biomarkers in 72 of 168 samples while the use of morphology and IHC detected 12 of those 72 biomarkers.
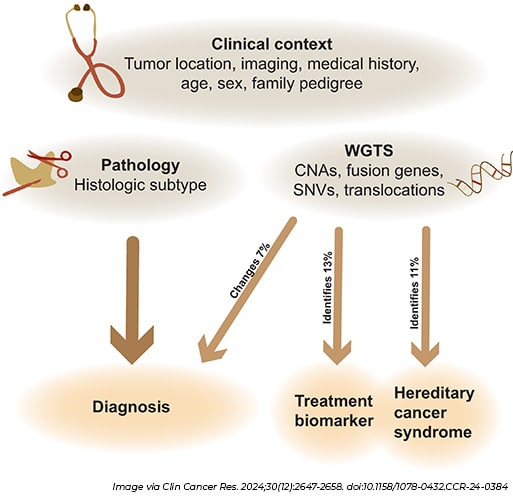
The authors of the study wrote that any genetic results produced by WGTS should still be evaluated in the context of findings from morphology, but this process could be used to help improve diagnosis once it becomes more cost effective and widely accessible.
Can T-cell Therapy Work for Solid Tumors?
The ability to identify specific biomarkers may have helped researchers discover a breakthrough treatment for advanced synovial sarcoma and myxoid round cell liposarcoma. T-cell therapy is a type of immunotherapy in which cells from a patient’s own blood are removed, genetically modified in some cases, multiplied, and then infused back into the patient. While this type of therapy has proven effective in blood cancers, the only solid tumor it has been approved to treat is melanoma. But that may soon change thanks to a T-cell therapy called afamitresgene autoleucel (afami-cel) designed to target melanoma-associated antigen A4 (MAGE-A4), a protein heavily expressed in patients who have a tumor with an HLA-A*02 genotype.
Results of the SPEARHEAD-1 phase II trial published in The Lancet found overall response rates of 39% for patients with synovial sarcoma and 25% for patients with myxoid round cell liposarcoma after a median follow-up time of 32.6 months. The 52 patients in the trial treated with afami-cel had been previously treated with two to four lines of therapy. The FDA granted afami-cel priority review for the treatment of advanced synovial sarcoma in February 2024 and a decision about approval is expected in August 2024.
Another set of researchers are taking a different approach by instead using T cells to serve as a delivery system for two therapies. In this case, they are engineering donor T cells rather than using a patient’s own T cells, which means it could potentially be produced at a lower cost and be made available off the shelf.
In a study published in Science Translational Medicine, the donor T cells were designed to release a synthetic antibody targeting GD2, which past studies revealed to be an immunotherapeutic target for neuroblastoma and osteosarcoma, as well as cytokines that would help to activate nearby immune cells in the tumor microenvironment. Further, zoledronic acid was used to help these T cells bind to the bone to enhance the effectiveness of the therapy by ensuring the treatment is delivered near the tumor site. When tested in mice, they found that osteosarcoma tumors failed to grow and decreased in size in eight of the 10 test subjects. The researchers plan to test this therapy next in human patients with secondary bone cancers.
Nanomedicine Combinations that Work Well Together
The idea behind the emerging field of “theranostics” is to combine therapeutics with diagnostics. When you add in the use of nanoparticles to deliver the treatment more precisely, then you have nanotheranostics. To test the use of this approach to treat osteosarcoma, researchers assembled a nanotheranostic platform using a layer-by-layer approach that incorporated chemotherapy drugs docetaxel and doxorubicin along with carbon quantum dots to serve as imagining nanoprobes to monitor whether these drugs were reaching their target. To help target the chemotherapies to the tumor cells, layers of aspartic acid-rich and YSAYPDSVPMMS peptides as well as the monoclonal antibody trastuzumab, which have previously been used to target osteosarcoma, were included in the platform.
When testing this nanotheranostic platform in two cell lines with osteosarcoma, the researchers saw almost complete cancer cell death in both models at every concentration of the chemotherapies they tested, according to results published in APL Bioengineering. While chemotherapy has already been shown to improve the prognosis of patients with non-metastatic osteosarcoma, patients often stop treatment due to drug resistance, toxicity, or side effects caused by the chemotherapy spreading to other parts of the body. The researchers wrote that this targeted model could help alleviate those side effects, but it still needs to be validated in humans.
A different nanomedicine combination was used to treat osteosarcoma and fibrosarcoma in a study published in Molecular Cancer Therapeutics, a journal of the AACR. In mouse models with these cancers, researchers pretreated the subjects with ketotifen, a type of asthma medication, in an attempt to reduce tumor stiffness and increase perfusion so therapy would be more effective. Then, they tested two forms of nanomedicine, one that included just doxorubicin and another that combined doxorubicin with alendronate, a drug used to treat osteoporosis and bone metastasis. Each of these was tested in combination with an anti-PD-1 immune checkpoint inhibitor, a type of immunotherapy that prevents cancer cells from turning on the “brakes” that stop immune cell activity.
Ultrasound imaging confirmed the reduction of tumor stiffness following ketotifen, and tumors were significantly smaller in all treatment groups. Of all the combinations, ketotifen along with the alendronate and doxorubicin nanomedicine and the anti-PD-1 resulted in the smallest tumors. The researchers have initiated a phase II trial in the European Union to validate these findings.


