We’re listening to Bobby Pickett’s “Monster Mash” this week and dishing out a spooky Halloween edition of our quarterly blog series, From the Bench, where we share some of the creative approaches bench scientists are using to study cancer. So, if you’re working in the lab late one night (or even if you’re not), keep away the monsters and other eerie sights Pickett sang about by reading how researchers are turning cancer’s tricks against itself, testing creepy crawly brain parasites for drug delivery, giving mice ghost-like transparency with a common food dye, and more.
Trick or Treat? Treating Cancer by Using its Trick Against Itself
Mitochondria are the powerhouses of the cell, and cancers have developed some sneaky tricks to keep themselves powered, including forming intercellular nanotubes to steal these energy-generating organelles from T cells. This tactic deals a double blow, simultaneously ramping up energy production in cancer cells and depleting energy from the very immune cells that seek to kill them. Studies have also shown that cancers can take mitochondria from surrounding stromal cells.
Now, researchers are turning the tables on cancer cells and using a similar strategy against them. In a study published in Cell, researchers developed an ex vivo platform to transplant mitochondria into extracted T cells prior to cell-based immunotherapy, with the hypothesis that this would prolong antitumor activity and delay exhaustion of the infused T cells.
They cocultured mouse T cells with bone marrow-derived stromal cells (BMSCs) and observed that this led to the formation of intercellular nanotubes and the transfer of mitochondria from BMSCs to T cells.
When the mitochondria-laden T cells were then infused into tumor-bearing mice, they had greater expansion, increased tumor infiltration, and fewer signs of T-cell exhaustion than T cells without transferred mitochondria. This resulted in better antitumor activity that significantly prolonged mouse survival. The authors proposed mitochondria transfer as a new potential therapeutic avenue.
Ghost-like Transparency in Living Mice
Why are ghosts often depicted as transparent? Living tissue is made of many components that refract light differently, causing the light to scatter instead of penetrating through layers of cells. Free of their corporeal forms, ghosts don’t have this issue; neither do mice that have been bathed in a surprisingly common dye.
In a recent study in Science, researchers showed that the food coloring tartrazine (yellow #5) could turn mouse skin transparent, allowing them to visualize internal organs with the naked eye. In essence, tartrazine synchronized the refractive indices of molecular components in the skin, reducing light scattering and allowing the light to pass through with minimal interference. By applying tartrazine to the abdomens of living mice, the researchers were able to perform real-time fluorescent imaging of cholinergic neurons in the mouse intestine and study their impact on gut motility without surgery. Further, they showed that the effects of tartrazine are reversible by simply rinsing the dye off the skin.
The researchers suggested that these results may help to identify other dyes with transparency-inducing properties to facilitate the real-time imaging of a variety of biological processes. But while tartrazine is found in many foods—almost certainly including your bucket of Halloween candy—the technology is not quite ready to be tested in humans.
Creepy Crawly Creatures to Deliver Therapeutics

The blood-brain barrier protects the brain from harmful substances and pathogens, but it also prevents the delivery of certain drugs and other therapeutics to brain tissue, including brain tumors. The protozoan parasite Toxoplasma gondii (T. gondii) can traverse this barrier, causing an infection known as toxoplasmosis.
In a recent study in Nature Microbiology, researchers engineered these creepy crawlies to secrete proteins of interest directly into brain cells. T. gondii uses two systems to secrete proteins into host cells: the rhoptries, through which T. gondii outside the cell can push proteins through the host cell membrane, and the dense granules, through which T. gondii inside the cell can secrete proteins directly into the cytosol. By fusing proteins of interest with proteins typically secreted through each of these pathways, the researchers showed that engineered T. gondii could successfully deliver target proteins to neurons in vitro using both secretion pathways and that those fusion proteins functioned as expected in host cells. Engineered T. gondii also trafficked to the brain and delivered fusion proteins in vivo.
The researchers noted that delivering enough protein to have a therapeutic effect would likely require them to boost the secretion capabilities of T. gondii or attenuate its infectiousness so more parasites can be delivered. While more research is required, the study authors expressed hope that these microscopic brain-dwellers can someday deliver therapeutics.
Striking a Deal With the Devil: Activating Oncogenic Signaling for Cancer Treatment
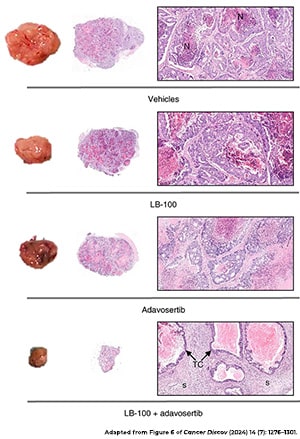
Shutting off cancer-promoting pathways is a common treatment strategy, but in a recent study published in the American Association for Cancer Research (AACR) journal Cancer Discovery, researchers hypothesized that activating these pathways may be an effective approach, too.
Their rationale was that hyperactivation of oncogenic signaling in cancer cells would exacerbate cellular stress and make the cells dependent on stress response pathways. This, they reasoned, would make the cancer cells susceptible to drugs that block stress responses.
They tested their hypothesis in colorectal cancer cell lines, treating the cells with an inhibitor of the tumor suppressor PP2A, which amplified the activation of multiple oncogenic pathways. When PP2A inhibition was combined with an inhibitor of the replication stress response protein WEE1, cancer cells were killed at a higher rate than observed with either inhibitor alone. The combination also exhibited antitumor activity in patient xenograft mouse models of colorectal cancer.
Moreover, the researchers found that when cancer cells became resistant to the combined inhibition of PP2A and WEE1, they did so by shutting off the oncogenic signaling that made them dependent on WEE1—the very signaling that was driving the cancer.
The researchers proposed hyperactivation of oncogenic signaling as a potential therapeutic approach that not only makes cancers vulnerable to WEE1 inhibition but also leads to an acquired resistance phenotype that effectively turns off tumor-driving pathways.
Robbing the Grave of Necrotic Tumors: A Novel Antibody-drug Conjugate Design
The nucleus is the cellular command center of cells, controlling which genes are turned on or off, among other important functions. Consequently, if there were a way to target the nuclei in cancer cells, we could manipulate their behavior and stop their progression.
Antibodies have long offered a way to target cancer cells with precision, and equipping them with anticancer drugs—in complexes known as antibody-drug conjugates—has proven a viable method of delivering chemotherapy directly to tumors. However, gaining access to the privileged nucleus is challenging.
In a clever new strategy recently published in ACS Central Science, researchers generated “antinuclear missile” antibodies that can stealthily hitch a ride into the nuclei of cancer cells. The antibodies achieve this by binding, in the blood, the building blocks of DNA that are released by dying cancer cells and ultimately taken back up by living cancer cells in the vicinity. Taking the approach a step further, the researchers modified these nucleus-infiltrating antibodies to act as Trojan horses, so that upon entry into the nucleus they are triggered to deliver a drug that then kills the cancer cell.
Furthermore, because this method of targeted drug delivery does not rely on surface markers, which may vary between different people and cancer types, this strategy could potentially be applied to treat any cancer type. And by incorporating payloads with immunomodulatory properties, this approach could enhance the effectiveness of immunotherapy and offer a wide variety of other potential applications in oncology.


