As winter marches on, let’s dive into the cool articles highlighted this month by the editors of the AACR journals. Grab a blanket and keep reading to learn how nicotine levels in e-cigarettes impact exposure to various toxins, why certain species are more likely to develop cancer, and results from a first-in-human trial evaluating a HER2/4-1BB bispecific molecule, among other topics. As always, the full text of each article is freely available for a limited time.
Journal: Blood Cancer Discovery
Minimal Residual Disease as an Early Endpoint for Accelerated Drug Approval in Myeloma: A Roadmap
Improvements in multiple myeloma treatments have extended patient survival to a decade or more. Treatment response rates >90% have introduced new challenges for drug development, including a need for early endpoints with greater sensitivity. The FDA, based on data from two independent academic research groups and industry, evaluated minimal residual disease (MRD) negativity as an intermediate endpoint for progression-free and overall survival, culminating in a unanimous vote by the Oncologic Drugs Advisory Committee in April 2024 supporting MRD-negative complete response as an early endpoint reasonably likely to predict clinical benefit in multiple myeloma that may be used to support accelerated approval.
Significance: The acceptance of MRD-negative complete response as an endpoint that is reasonably likely to predict clinical benefit will allow for the design of streamlined clinical trials for accelerated approval, enabling significantly faster patient access to novel therapies. Cooperative efforts were required to obtain and analyze clinical trial data from multiple sponsors and to determine the best approach to analysis with a relatively limited number of available datasets. The process to evaluate MRD as an intermediate endpoint, undertaken jointly by myeloma researchers and industry, with feedback from the FDA, serves as a roadmap for other areas of oncology to develop intermediate endpoints.
Journal: Cancer Discovery
Cancer Prevalence across Vertebrates
Cancer is pervasive across multicellular species, but what explains the differences in cancer prevalence across species? Using 16,049 necropsy records for 292 species spanning three clades of tetrapods (amphibians, sauropsids, and mammals), we found that neoplasia and malignancy prevalence increases with adult mass (contrary to Peto’s paradox) and somatic mutation rate but decreases with gestation time. The relationship between adult mass and malignancy prevalence was only apparent when we controlled for gestation time. Evolution of cancer susceptibility appears to have undergone sudden shifts followed by stabilizing selection. Outliers for neoplasia prevalence include the common porpoise (<1.3%), the Rodrigues fruit bat (<1.6%), the black-footed penguin (<0.4%), ferrets (63%), and opossums (35%). Discovering why some species have particularly high or low levels of cancer may lead to a better understanding of cancer syndromes and novel strategies for the management and prevention of cancer.
Significance: Evolution has discovered mechanisms for suppressing cancer in a wide variety of species. By analyzing veterinary necropsy records, we can identify species with exceptionally high or low cancer prevalence. Discovering the mechanisms of cancer susceptibility and resistance may help improve cancer prevention and explain cancer syndromes.
A related commentary was published in the January issue, and the study was also featured on the issue’s cover. Read more about this study and its implications for human cancer development in a recent blog post.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Biomarkers of Nicotine and Toxicant Exposure by E-Liquid Nicotine Concentration Level among US Adult Exclusive E-Cigarette Users
Background: The current e-cigarette market has been rapidly evolving with an increase in the share of high nicotine concentration vaping products. This study examined urinary biomarkers of exposure (BOE) by nicotine concentration level among exclusive e-cigarette users.
Methods: Data were drawn from wave 5 (December 2018–November 2019) of the Population Assessment of Tobacco and Health study. Between-subject differences in BOEs of nicotine, metal, tobacco-specific nitrosamine, and volatile organic compounds were examined across e-cigarettes containing nicotine or not [yes (n = 300) vs. no (n = 31) vs. non-tobacco use (n = 3,021)] and different nicotine concentration levels (0.1%–1.7%, 1.8%–4.9%, and 5.0%+).
Results: Among 3,353 participants, exclusive e-cigarette users exhibited higher mean concentrations of nicotine metabolites than non-tobacco users. Nicotine e-cigarette users had higher concentrations of total nicotine equivalents-2 [TNE2; mean (95% confidence interval), 21.8 (15.2–31.2) vs. 0.2 (0.1–0.6) nmol/mg creatinine, P < 0.0001] and cotinine [1,418.2 (998.0–2,015.4) vs. 12.2 (0.1–0.6), P < 0.0001) ng/mg creatinine, P < 0.0001] than non-nicotine e-cigarette users. Users of e-cigarette products with nicotine levels of 1.8% to 4.9% had higher TNE2 and cotinine levels than those using 0.1% to 1.7%, though differences were insignificant after adjusting for covariates. As compared to non-tobacco users, nicotine vapers had higher concentrations of lead (adjusted P = 0.01).
Conclusions: Nicotine containing e-cigarette users exhibited elevated levels of nicotine metabolites than non-nicotine containing vapers and non-tobacco users. Future research needs to investigate health effects of e-cigarette use across different nicotine levels.
Impact: Regulating the nicotine content in e-cigarettes could be crucial in managing nicotine exposure and potentially mitigating associated health risks.
Journal: Cancer Immunology Research
CD27-Armored BCMA CAR T-cell Therapy (CBG-002) for Relapsed and Refractory Multiple Myeloma: A Phase I Clinical Trial
B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy has been approved for the treatment of relapsed and refractory multiple myeloma (RRMM); however, whether patients have long-term responses has yet to be established. We investigated the feasibility of CBG-002, a CD27-armored BCMA CAR T-cell therapy, to improve clinical efficacy in patients with RRMM. We present preclinical data showing the activity of CBG-002 against myeloma and results from a phase I clinical trial (NCT04706936) evaluating its safety and efficacy in patients with RRMM. The primary endpoint was safety, as assessed by grade 3 or 4 adverse events (AE). Key secondary endpoints were overall response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). A total of 11 patients were enrolled and received CBG-002 therapy. Nine patients developed grade 1 or 2 cytokine release syndrome (CRS), whereas no patients experienced grade 3 or higher CRS or immune effector cell–associated neurotoxicity syndrome. Other grade 3 or higher AEs included neutropenia (72.7%), thrombocytopenia (45.5%), and anemia (36.4%). At a median follow-up of 16.7 months, the ORR was 81.8%, including a stringent complete response/complete response rate of 45.5%, very good partial response rate of 18.2%, and partial response rate of 18.2%, with a median DOR of 8.9 (range 1.8–21.9) months. The median OS was not reached, and the median PFS was 8.5 (2.7–22.9) months. In this phase I study, CBG-002, a CD27-armored BCMA CAR T-cell therapy, demonstrated safety and clinical efficacy in patients with RRMM.
Journal: Cancer Prevention Research
Phase II Clinical Chemoprevention Trial of Weekly Erlotinib before Bladder Cancer Surgery
We performed a clinical trial in patients with non–muscle-invasive (NMI) urothelial cancer randomized (2:1) to the EGFR tyrosine kinase inhibitor erlotinib or placebo (either orally once weekly × 3 doses prior to scheduled surgery) to assess for a difference in EGFR phosphorylation in tumor-adjacent normal urothelium <24 hours post–study dose and tolerance of weekly erlotinib therapy. Thirty-seven volunteers (6 female/31 male; mean age 70; 35 White/2 non-White) with confirmed or suspected NMI urothelial cancer were enrolled into either erlotinib (n = 24; 900 mg-13, 600 mg-11) or placebo (n = 13). IHC assessment of phosphorylated and total EGFR in tumor-adjacent normal urothelium (20 erlotinib and 9 placebo subjects) or tumor (21 erlotinib and 11 placebo subjects) at study end showed no significant difference between those receiving erlotinib or placebo. This was also true for other assessed tissue biomarkers (phosphorylated ERK, ERK, E-cadherin, p53, and Ki67). Adverse events were more common, in a dose-related fashion, in participants receiving erlotinib, e.g., 38% experienced grade 1 with rare grade 2 diarrhea and skin toxicity versus 8% in placebo. Clinically insignificant but statistically significant (P = 0.001) elevations in serum total bilirubin and creatinine were observed in participants receiving erlotinib. Serum erlotinib and metabolite concentrations (OSI-420) confirmed compliance in all subjects receiving erlotinib and did not significantly differ between the 600 and 900 mg doses. Despite compelling preclinical and clinical data for targeted EGFR inhibition in bladder cancer prevention, these data do not support the use of weekly erlotinib therapy to prevent progression of NMI bladder cancer.
Prevention Relevance: We evaluated the potential of erlotinib in preventing cancer by performing a randomized, double-blind, placebo-controlled trial of weekly erlotinib therapy in participants undergoing surgical removal of suspected noninvasive bladder neoplasia. Weekly erlotinib therapy was tolerated with common grade 1 to 2 toxicities but without evidence of beneficial effect upon urothelial tissue.
A related commentary was published in the January issue.
Journal: Cancer Research (January 1 issue)
H4K20me3-Mediated Repression of Inflammatory Genes Is a Characteristic and Targetable Vulnerability of Persister Cancer Cells
Anticancer therapies can induce cellular senescence or drug-tolerant persistence, two types of proliferative arrest that differ in their stability. While senescence is highly stable, persister cells efficiently resume proliferation upon therapy termination, resulting in tumor relapse. Here, we used an ATP-competitive mTOR inhibitor to induce and characterize persistence in human cancer cells of various origins. Using this model and previously described models of senescence, we compared the same cancer cell lines under the two types of proliferative arrest. Persister and senescent cancer cells shared an expanded lysosomal compartment and hypersensitivity to BCL-XL inhibition. However, persister cells lacked other features of senescence, such as loss of lamin B1, senescence-associated β-galactosidase activity, upregulation of MHC-I, and an inflammatory and secretory phenotype (senescence-associated secretory phenotype or SASP). A genome-wide CRISPR/Cas9 screening for genes required for the survival of persister cells revealed that they are hypersensitive to the inhibition of one-carbon (1C) metabolism, which was validated by the pharmacologic inhibition of serine hydroxymethyltransferase, a key enzyme that feeds methyl groups from serine into 1C metabolism. Investigation into the relationship between 1C metabolism and the epigenetic regulation of transcription uncovered the presence of the repressive heterochromatic mark H4K20me3 at the promoters of SASP and IFN response genes in persister cells, whereas it was absent in senescent cells. Moreover, persister cells overexpressed the H4K20 methyltransferases KMT5B/C, and their downregulation unleashed inflammatory programs and compromised the survival of persister cells. In summary, this study identifies distinctive features and actionable vulnerabilities of persister cancer cells and provides mechanistic insight into their low inflammatory activity.
Significance: Cell persistence and senescence are distinct states of proliferative arrest induced by cancer therapy, with persister cells being characterized by the silencing of inflammatory genes through the heterochromatic mark H4K20me3.
A related commentary was published in the January 1 issue, and the study was also featured on the issue’s cover.
Journal: Cancer Research (January 15 issue)
FOXR2 Targets LHX6+/DLX+ Neural Lineages to Drive Central Nervous System Neuroblastoma
Central nervous system neuroblastoma with forkhead box R2 (FOXR2) activation (NB-FOXR2) is a high-grade tumor of the brain hemispheres and a newly identified molecular entity. Tumors express dual neuronal and glial markers, leading to frequent misdiagnoses, and limited information exists on the role of FOXR2 in their genesis. To identify their cellular origins, we profiled the transcriptomes of NB-FOXR2 tumors at the bulk and single-cell levels and integrated these profiles with large single-cell references of the normal brain. NB-FOXR2 tumors mapped to LHX6+/DLX+ lineages derived from the medial ganglionic eminence, a progenitor domain in the ventral telencephalon. In vivo prenatal Foxr2 targeting to the ganglionic eminences in mice induced postnatal cortical tumors recapitulating human NB-FOXR2–specific molecular signatures. Profiling of FOXR2 binding on chromatin in murine models revealed an association with ETS transcriptional networks, as well as direct binding of FOXR2 at key transcription factors that coordinate initiation of gliogenesis. These data indicate that NB-FOXR2 tumors originate from LHX6+/DLX+ interneuron lineages, a lineage of origin distinct from that of other FOXR2-driven brain tumors, highlight the susceptibility of ventral telencephalon–derived interneurons to FOXR2-driven oncogenesis, and suggest that FOXR2-induced activation of glial programs may explain the mixed neuronal and oligodendroglial features in these tumors. More broadly, this work underscores systematic profiling of brain development as an efficient approach to orient oncogenic targeting for in vivo modeling, critical for the study of rare tumors and development of therapeutics.
Significance: Profiling the developing brain enabled rationally guided modeling of FOXR2-activated CNS neuroblastoma, providing a strategy to overcome the heterogeneous origins of pediatric brain tumors that hamper tumor modeling and therapy development.
A related commentary was published in the January 15 issue.
Journal: Clinical Cancer Research (January 1 issue)
Phase I/II Investigator-Initiated Study of Olaparib and Temozolomide in SCLC: Final Analysis and CNS Outcomes
Purpose: Temozolomide plus PARP inhibition has shown promise in small cell lung cancer (SCLC). We previously reported outcomes from the first 50 patients (cohort 1) of a phase I/II trial of olaparib/temozolomide in recurrent SCLC. In this study, we report a final analysis of this trial, including a second cohort with an alternate dosing strategy and an exploratory analysis of central nervous system (CNS)–specific outcomes.
Patients and Methods: This was an open-label phase I/II trial testing the combination of olaparib and temozolomide in relapsed SCLC. The primary endpoint was objective response rate (ORR). Secondary endpoints were safety, progression-free survival, and overall survival. We tested escalating doses of olaparib/temozolomide across two cohorts, both of which had temozolomide dosed on days 1 to 7 of each 21-day cycle. In previously published cohort 1, olaparib was dosed on days 1 to 7; in cohort 2, olaparib was dosed continuously.
Results: Sixty-six patients were enrolled across the two cohorts: 50 in cohort 1 and 16 in cohort 2. The confirmed ORR of cohort 1 was 41.7% (20/48 evaluable), and the confirmed ORR of cohort 2 was 7% (1/14 evaluable; closed after dose escalation to enrollment for lack of observed efficacy). Among 15/66 patients (22.7%) with untreated brain metastases at enrollment, the best overall intracranial response was complete response in 6/15 patients, partial response in 4/15 patients, and stable disease in 3/15 patients for a CNS disease control rate of 87% (95% confidence interval, 59.5%–98.3%).
Conclusions: Olaparib/temozolomide may be effective in relapsed SCLC, especially for patients with CNS disease. Ongoing analyses with regard to optimal dosing schedule will inform potential for future use of this combination in SCLC.
Journal: Clinical Cancer Research (January 15 issue)
A First-in-Human Study of Cinrebafusp Alfa, a HER2/4-1BB Bispecific Molecule, in Patients with HER2-Positive Advanced Solid Malignancies
Purpose: 4-1BB (CD137) is a costimulatory immune receptor expressed on activated T cells, activated B cells, NK cells, and tumor-infiltrating lymphocytes, making it a promising target for cancer immunotherapy. Cinrebafusp alfa, a monoclonal antibody-like bispecific protein targeting HER2 and 4-1BB, aims to localize 4-1BB activation to HER2-positive tumors. This study evaluated the safety, tolerability, and preliminary efficacy of cinrebafusp alfa in patients with previously treated HER2-positive malignancies.
Patients and Methods: This was a multicenter dose-escalation study involving patients with HER2-positive malignancies who received prior treatment. The study assessed the safety and efficacy of cinrebafusp alfa across various dose levels. Patients were assigned to different cohorts, and antitumor responses were evaluated. The study aimed to determine the MTD and to observe any clinical activity at different dose levels.
Results: Of 40 evaluable patients in the “active dose” efficacy cohorts, five showed an antitumor response, resulting in an overall response rate of 12.5% and a disease-control rate of 52.5%. Clinical activity was observed at the 8 and 18 mg/kg dose levels, with confirmed objective response rates of 28.6% and 25.0%, respectively. Cinrebafusp alfa was safe and tolerable, with grade ≤2 infusion-related reactions being the most frequent treatment-related adverse event. MTD was not reached during the study.
Conclusions: Cinrebafusp alfa demonstrates promising activity in patients with HER2-positive malignancies who have progressed on prior HER2-targeting regimens. Its acceptable safety profile suggests it could be a treatment option for patients not responding to existing HER2-directed therapies.
A related commentary was published in the January 15 issue.
Journal: Molecular Cancer Research
N-Linked Fucosylated Glycans Are Biomarkers for Prostate Cancer with a Neuroendocrine and Metastatic Phenotype
Prostate cancer is a heterogeneous disease with a spectrum of pathology and outcomes ranging from indolent to lethal. Although there have been recent advancements in prognostic tissue biomarkers, limitations still exist. We leveraged matrix-assisted laser desorption/ionization imaging of formalin-fixed, paraffin embedded prostate cancer specimens to determine if N-linked glycans expressed in the extracellular matrix of lethal neuroendocrine prostate cancer were also expressed in conventional prostate adenocarcinomas that were associated with poor outcomes. We found that N-glycan fucosylation was abundant in neuroendocrine prostate cancer as well as adenocarcinomas at the time of prostatectomy that eventually developed recurrent metastatic disease. Analysis of patient-derived xenografts revealed that this fucosylation signature was enriched differently across metastatic disease organ sites, with the highest abundance in liver metastases. These data suggest that N-linked fucosylated glycans could be an early tissue biomarker for poor prostate cancer outcomes.
Implications: These studies identify that hyper-fucosylated N-linked glycans are enriched in neuroendocrine prostate cancer and conventional prostate adenocarcinomas that progress to metastatic disease, thus advancing biomarker discovery and providing insights into mechanisms underlying metastatic disease.
Journal: Molecular Cancer Therapeutics
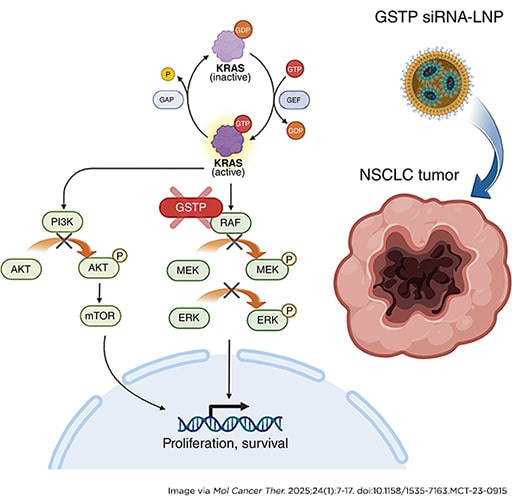
A Novel Lipid Nanoparticle NBF-006 Encapsulating Glutathione S-Transferase P siRNA for the Treatment of KRAS-Driven Non–small Cell Lung Cancer
Non–small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers, and KRAS mutations occur in 25% to 30% of NSCLC. Our approach to developing a therapeutic with the potential to target KRAS-mutant NSCLC was to identify a new target involved in modulating signaling proteins in the RAS pathway. Glutathione S-transferase P (GSTP), known as a phase II detoxification enzyme, has more recently been identified as a modulator of MAPK-related cell signaling pathways. Therefore, developing a GSTP siRNA may be an effective therapeutic approach to treat KRAS-mutant NSCLC. The lead drug product candidate (NBF-006) is a proprietary siRNA-based lipid nanoparticle comprising GSTP siRNA (NDT-05-1040). Here, studies using a panel of KRAS-mutant NSCLC cell lines demonstrated that NDT-05-1040 is a very potent and selective GSTP siRNA inhibitor. Our Western blot analysis showed that NDT-05-1040 effectively decreased the phosphorylation of MAPK and PI3K pathway components while upregulating apoptotic signaling cascade. Our in vivo studies revealed statistically significant higher distribution of NBF-006 to the lungs and tumor as compared with the liver. In the subcutaneous and orthotopic tumor models, NBF-006 led to a statistically significant and dose-dependent antitumor growth inhibition. Furthermore, quantitative image analysis of proliferating cell nuclear antigen and PARP staining showed that NBF-006 decreased proliferation and induced apoptosis, respectively, in tumors. Additionally, in a surgically implanted orthotopic lung tumor model, the survival rate of the NBF-006 treatment group was significantly prolonged (P < 0.005) as compared with the vehicle control group. Together, these preclinical studies supported advancement of NBF-006 into clinical studies.
This article was featured on the cover of the January issue.
Journal: Cancer Research Communications
Dynamic Evolution of Fibroblasts Revealed by Single-Cell RNA Sequencing of Human Pancreatic Cancer
Cancer progression and response to therapy are inextricably reliant on the coevolution of a supportive tissue microenvironment. This is particularly evident in pancreatic ductal adenocarcinoma, a tumor type characterized by expansive and heterogeneous stroma. Herein, we employed single-cell RNA sequencing and spatial transcriptomics of normal, inflamed, and malignant pancreatic tissues to contextualize stromal dynamics associated with disease and treatment status, identifying temporal and spatial trajectories of fibroblast differentiation. Using analytical tools to infer cellular communication, together with a newly developed assay to annotate genomic alterations in cancer cells, we additionally explored the complex intercellular networks underlying tissue circuitry, highlighting a fibroblast-centric interactome that grows in strength and complexity in the context of malignant transformation. Our study yields new insights on the stromal remodeling events favoring the development of a tumor-supportive microenvironment and provides a powerful resource for the exploration of novel points of therapeutic intervention in pancreatic ductal adenocarcinoma.
Significance: Pancreatic cancer remains a high unmet medical need. Understanding the interactions between stroma and cancer cells in this disease may unveil new opportunities for therapeutic intervention.


