As Halloween approaches, Cancer Research Catalyst brings you no tricks and all treats in the October edition of editors’ picks. This month, the editors of the 10 American Association for Cancer Research (AACR) journals selected a head-to-head comparison of two CAR T therapies for lymphoma, a new type of blood test for colorectal cancer detection, two phase II clinical trials, and more.
Like your Halloween candy, these studies are freely available for a limited time.
Journal: Blood Cancer Discovery
A Multicenter Real-life Prospective Study of Axicabtagene Ciloleucel versus Tisagenlecleucel Toxicity and Outcomes in Large B-cell Lymphomas
This real-world prospective observational study across 21 Italian centers (CART-SIE) compares axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) outcomes in 485 patients with relapsed/refractory large B-cell lymphoma with baseline characteristics matched by stabilized inverse propensity score weighting. Axi-cel versus tisa-cel had higher all-grade cytokine release syndrome (78.6% vs. 89.3%, P = 0.0017) and neurotoxicity (9.9% vs. 32.2%, P < 0.0001) but also superior progression-free survival (PFS) at 1 year (46.5% vs. 34.1%, P = 0.0009). Even among patients who failed bridging therapy, axi-cel PFS was superior to tisa-cel (37.5% vs. 22.7%, P = 0.0059). Differences in overall survival and high-grade immune toxicities were not significant. The CAR-HEMATOTOX score not only predicted hematologic toxicity but also 1-year survival outcomes (51.5% in CAR-HEMATOTOX high vs. 77.2% in CAR-HEMATOTOX low, P < 0.0001). Twenty patients developed second primary malignancies, including two cases of T-cell neoplasms. These findings enable more informed selection of anti-CD19 CAR T-cell therapy, balancing bridging, safety, and efficacy considerations for individual patients.
Significance: The findings of this study on 485 patients with relapsed/refractory large B-cell lymphoma treated with commercial axi-cel and tisa-cel indicate axi-cel’s superior PFS after propensity score weighting. The predictive utility of CAR-HEMATOTOX in assessing not only toxicity but also outcomes across both CAR T-cell products may guide future risk-stratified management strategies.
Journal: Cancer Discovery
The History of Chromosomal Instability in Genome-Doubled Tumors
Tumors frequently display high chromosomal instability and contain multiple copies of genomic regions. Here, we describe Gain Route Identification and Timing In Cancer (GRITIC), a generic method for timing genomic gains leading to complex copy number states, using single-sample bulk whole-genome sequencing data. By applying GRITIC to 6,091 tumors, we found that non-parsimonious evolution is frequent in the formation of complex copy number states in genome-doubled tumors. We measured chromosomal instability before and after genome duplication in human tumors and found that late genome doubling was followed by an increase in the rate of copy number gain. Copy number gains often accumulate as punctuated bursts, commonly after genome doubling. We infer that genome duplications typically affect the landscape of copy number losses, while only minimally impacting copy number gains. In summary, GRITIC is a novel copy number gain timing framework that permits the analysis of copy number evolution in chromosomally unstable tumors.
Significance: Complex genomic gains are associated with whole-genome duplications, which are frequent across tumors, span a large fraction of their genomes, and are linked to poorer outcomes. GRITIC infers when these gains occur during tumor development, which will help to identify the genetic events that drive tumor evolution.
A related commentary was published in the September issue, and this article was featured on the cover.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Blood Magnesium Level and Risk of Hepatocellular Carcinoma in a Prospective Liver Cirrhosis Cohort
Background: Higher magnesium intake was linked to a lower risk of hepatocellular carcinoma (HCC). However, the relationship between blood magnesium level and HCC has not been fully characterized, especially among patients with liver cirrhosis who are at a higher risk for HCC.
Methods: In the Mass General Brigham Biobank, we developed a new prospective cohort of 1,430 patients with liver cirrhosis without liver cancer history using the validated International Classification of Diseases codes. We used Cox proportional hazards models to generate hazard ratios (HRs) with 95% confidence intervals (CI) for incident HCC and used generalized estimating equations to compare changes in liver biomarkers according to baseline blood magnesium, adjusting for age, sex, race, lifestyles, body mass index, type 2 diabetes, model for end-stage liver disease score, and hepatitis infection.
Results: During a median follow-up period of 4.26 years, 109 patients developed HCC. Magnesium deficiency (<1.70 mg/dL; N = 158) was associated with a higher risk of HCC (HR = 1.93; 95% CI, 1.12–3.30) compared with magnesium sufficiency (≥1.70 mg/dL; N = 1282). This association remained robust in the 1-year lag analysis (HR = 2.18; 95% CI, 1.11–4.28) and in sensitivity analysis excluding patients with alcoholic liver disease (HR = 2.41; 95% CI, 1.23–4.74). Magnesium in the lowest quartile was associated with a faster increase in alanine transaminase (β = 4.35; 95% CI, 1.06–7.63), aspartate aminotransferase (β = 6.46; 95% CI, 0.28–12.6), direct bilirubin (β = 0.18; 95% CI, 0.01–0.35), and total bilirubin (β = 0.21; 95% CI, 0.03–0.39), compared with the highest quartile.
Conclusions: Lower blood magnesium level is associated with higher HCC risk and unfavorable liver biomarker changes.
Impact: If confirmed, our findings may potentially enable better identification of high-risk patients for HCC and inform better management strategies for liver cirrhosis.
This study was highlighted in the October issue.
Journal: Cancer Immunology Research
Targeting Tumor-Associated Sialic Acids Using Chimeric Switch Receptors Based on Siglec-9 Enhances the Antitumor Efficacy of Engineered T Cells
Cancer exploits different mechanisms to escape T-cell immunosurveillance, including overexpression of checkpoint ligands, secretion of immunosuppressive molecules, and aberrant glycosylation. Herein, we report that IFNγ, a potent immunomodulator secreted in the tumor microenvironment, can induce α2,6 hypersialylation in cancer cell lines derived from various histologies. We focused on Siglec-9, a receptor for sialic acid moieties, and demonstrated that the Siglec-9+ T-cell population displayed reduced effector function. We speculated that Siglec-9 in primary human T cells can act as a checkpoint molecule and demonstrated that knocking out Siglec-9 using a clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system enhanced the functionality of primary human T cells. Finally, we aimed to augment cancer-specific T-cell activity by taking advantage of tumor hypersialylation. Thus, we designed several Siglec-9–based chimeric switch receptors (CSR), which included an intracellular moiety derived from costimulatory molecules (CD28/41BB) and different hinge regions. In an antigen-specific context, T cells transduced with Siglec-9 CSRs demonstrated increased cytokine secretions and upregulation of activation markers. Moreover, T cells equipped with specific Siglec-9 CSRs mediated robust antitumor activity in a xenograft model of human tumors. Overall, this work sheds light on tumor evasion mechanisms mediated by sialylated residues and exemplifies an approach to improve engineered T cell–based cancer treatment.
A related commentary was published in the October issue.
Journal: Cancer Prevention Research
Preventive Treatment with a CD73 Small Molecule Inhibitor Enhances Immune Surveillance in K-Ras Mutant Pancreatic Intraepithelial Neoplasia
Immunoprevention is an emerging consideration for solid tumors, including pancreatic ductal adenocarcinoma (PDAC). We and others have shown that Kras mutations in genetic models of spontaneous pancreatic intraepithelial neoplasia (PanIN), which is a precursor to PDAC, results in CD73 expression in the neoplastic epithelium and some populations of infiltrating immune cells, including macrophages and CD8 T cells. CD73 is an ecto-enzyme that converts extracellular adenosine monophosphate to adenosine, a critical immune inhibitory molecule in PDAC. We hypothesized inhibition of CD73 would reduce the incidence of PanIN formation and alter the immune microenvironment. To test our hypothesis, we used the KrasG12D; PdxCre1 (KC) genetically engineered mouse model and tested the utility of AB-680, a small molecule inhibitor targeting CD73, to inhibit PanIN progression. AB-680, or vehicle control, was administered using oral gavage delivery 3 days/week at 10 mg/kg, beginning when the mice were 2 months old and lasting 3 months. We euthanized the mice at 5 months old. In the KC model, we quantified significantly less pancreatitis, early and advanced PanIN, and quantified a significant increase in M1 macrophages in AB-680-treated mice. Single-cell RNA sequencing (scRNA-seq) of pancreata of AB-680-treated mice revealed increased infiltration of CD4+ T cells, CD8+ T cells, and mature B cells. The scRNA-seq analysis showed that CD73 inhibition reduced M2 macrophages, acinar, and PanIN cell populations. CD73 inhibition enhanced immune surveillance and expanded unique clonotypes of TCR and BCR, indicating that inhibition of CD73 augments adaptive immunity early in the neoplastic microenvironment.
Prevention Relevance: Previous studies found PanIN lesions in healthy pancreata. Not all progress to PDAC, suggesting a window for enhanced antitumor immunity through immunoprevention therapy. CD73 inhibition in our study prevents PanIN progression, reduces immune-suppressive macrophages and expands TCR and BCR unique clonotypes, highlighting an encouraging therapeutic avenue for high-risk individuals.
This study was featured on the cover of the October issue.
Journal: Cancer Research (October 1 issue)
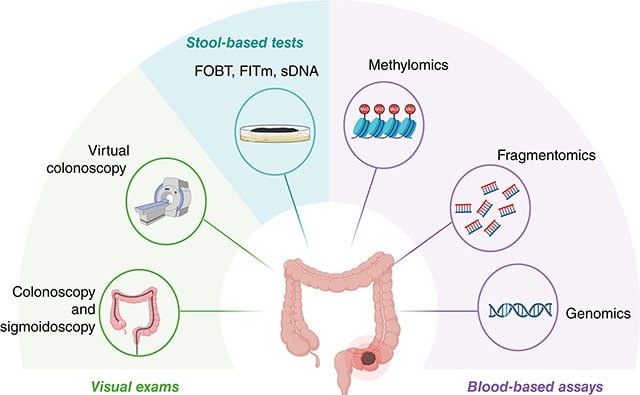
Multidimensional Fragmentomics Enables Early and Accurate Detection of Colorectal Cancer
Colorectal cancer is frequently diagnosed in advanced stages, highlighting the need for developing approaches for early detection. Liquid biopsy using cell-free DNA (cfDNA) fragmentomics is a promising approach, but the clinical application is hindered by complexity and cost. This study aimed to develop an integrated model using cfDNA fragmentomics for accurate, cost-effective early-stage colorectal cancer detection. Plasma cfDNA was extracted and sequenced from a training cohort of 360 participants, including 176 patients with colorectal cancer and 184 healthy controls. An ensemble stacked model comprising five machine learning models was employed to distinguish patients with colorectal cancer from healthy controls using five cfDNA fragmentomic features. The model was validated in an independent cohort of 236 participants (117 patients with colorectal cancer and 119 controls) and a prospective cohort of 242 participants (129 patients with colorectal cancer and 113 controls). The ensemble stacked model showed remarkable discriminatory power between patients with colorectal cancer and controls, outperforming all base models and achieving a high area under the receiver operating characteristic curve of 0.986 in the validation cohort. It reached 94.88% sensitivity and 98% specificity for detecting colorectal cancer in the validation cohort, with sensitivity increasing as the cancer progressed. The model also demonstrated consistently high accuracy in within-run and between-run tests and across various conditions in healthy individuals. In the prospective cohort, it achieved 91.47% sensitivity and 95.58% specificity. This integrated model capitalizes on the multiplex nature of cfDNA fragmentomics to achieve high sensitivity and robustness, offering significant promise for early colorectal cancer detection and broad patient benefit.
Significance: The development of a minimally invasive, efficient approach for early colorectal cancer detection using advanced machine learning to analyze cfDNA fragment patterns could expedite diagnosis and improve treatment outcomes for patients.
A related commentary about blood tests for colorectal cancer detection was published in the October 1 issue.
Journal: Cancer Research (October 15 issue)
A Genomics-Driven Artificial Intelligence–Based Model Classifies Breast Invasive Lobular Carcinoma and Discovers CDH1 Inactivating Mechanisms
Artificial intelligence (AI) systems can improve cancer diagnosis, yet their development often relies on subjective histologic features as ground truth for training. Herein, we developed an AI model applied to histologic whole-slide images using CDH1 biallelic mutations, pathognomonic for invasive lobular carcinoma (ILC) in breast neoplasms, as ground truth. The model accurately predicted CDH1 biallelic mutations (accuracy = 0.95) and diagnosed ILC (accuracy = 0.96). A total of 74% of samples classified by the AI model as having CDH biallelic mutations but lacking these alterations displayed alternative CDH1 inactivating mechanisms, including a deleterious CDH1 fusion gene and noncoding CDH1 genetic alterations. Analysis of internal and external validation cohorts demonstrated 0.95 and 0.89 accuracy for ILC diagnosis, respectively. The latent features of the AI model correlated with human-explainable histopathologic features. Taken together, this study reports the construction of an AI algorithm trained using a genetic rather than histologic ground truth that can robustly classify ILCs and uncover CDH1 inactivating mechanisms, providing the basis for orthogonal ground truth utilization for development of diagnostic AI models applied to whole-slide image.
Significance: Genetic alterations linked to strong genotypic–phenotypic correlations can be utilized to develop AI systems applied to pathology that facilitate cancer diagnosis and biologic discoveries.
Journal: Clinical Cancer Research (October 1 issue)
First-Line Anlotinib Treatment for Soft-Tissue Sarcoma in Chemotherapy-Ineligible Patients: An Open-Label, Single-Arm, Phase 2 Clinical Trial
Purpose: Standard treatment for patients with unresectable locally advanced or metastatic soft-tissue sarcoma (LA/M STS) is chemotherapy based on anthracyclines, but patient tolerance of chemotherapy is limited. The present trial (NCT03792542) investigated the use of anlotinib as first-line treatment for patients with advanced STS, in particular liposarcoma.
Patients and Methods: Eligible patients were previously untreated, pathologically confirmed, unresectable LA/M STS cases. Anlotinib was given orally at a dose of 12 mg once daily from days 1 to 14 every 3 weeks until disease progression or intolerable adverse events (AE) occurred. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were overall survival (OS), objective response rate, and disease control rate (DCR). The safety profile was also evaluated.
Results: Forty patients were enrolled from April 2019 to June 2022 and are included in the intention-to-treat analysis. The median PFS was 6.83 months [95% confidence interval (CI), 4.17–8.71] and the median OS 27.40 months (95% CI, 16.43–not evaluable); 1 patient reached partial response and 26 attained stable disease, with a DCR of 67.5% (27/40). Median PFS and OS times for liposarcoma patients were 8.71 and 16.23 months, respectively. Ten (25.0%) patients had treatment-related AEs ≥ grade 3, with in particular a higher incidence of hypertension (15.0%) and proteinuria (7.5%).
Conclusions: The findings suggest a potential benefit in using front-line anlotinib to treat patients with STS, who are not eligible for cytotoxic chemotherapy. Of note, the clinical outcomes for the liposarcoma subgroup of patients were encouraging.
A related commentary was published in the October 1 issue.
Journal: Clinical Cancer Research (October 15 issue)
Evaluating Debio 1347 in Patients with FGFR Fusion-Positive Advanced Solid Tumors from the FUZE Multicenter, Open-Label, Phase II Basket Trial
Purpose: This multicenter phase II basket trial investigated the efficacy, safety, and pharmacokinetics of Debio 1347, an investigational, oral, highly selective, ATP-competitive, small molecule inhibitor of FGFR1–3, in patients with solid tumors harboring a functional FGFR1–3 fusion.
Patients and Methods: Eligible adults had a previously treated locally advanced (unresectable) or metastatic biliary tract (cohort 1), urothelial (cohort 2), or another histologic cancer type (cohort 3). Debio 1347 was administered at 80 mg once daily, continuously, in 28-day cycles. The primary endpoint was the objective response rate. Secondary endpoints included duration of response, progression-free survival, overall survival, pharmacokinetics, and incidence of adverse events.
Results: Between March 22, 2019, and January 8, 2020, 63 patients were enrolled and treated, 30 in cohort 1, 4 in cohort 2, and 29 in cohort 3. An unplanned preliminary statistical review showed that the efficacy of Debio 1347 was lower than predicted, and the trial was terminated. In total, 3 of 58 evaluable patients had partial responses, representing an objective response rate of 5%, with a further 26 (45%) having stable disease (≥6 weeks duration). Grade ≥3 treatment-related adverse events occurred in 22 (35%) of 63 patients, with the most common being hyperphosphatemia (13%) and stomatitis (5%). Two patients (3%) discontinued treatment due to adverse events.
Conclusions: Debio 1347 had manageable toxicity; however, the efficacy in patients with tumors harboring FGFR fusions did not support further clinical evaluation in this setting. Our transcriptomic-based analysis characterized in detail the incidence and nature of FGFR fusions across solid tumors.
A related commentary was published in the October 15 issue.
Journal: Molecular Cancer Research
NAPRT Silencing in FH-Deficient Renal Cell Carcinoma Confers Therapeutic Vulnerabilities via NAD+ Depletion
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) is caused by loss of function mutations in fumarate hydratase (FH) and results in an aggressive subtype of renal cell carcinoma with limited treatment options. Loss of FH leads to accumulation of fumarate, an oncometabolite that disrupts multiple cellular processes and drives tumor progression. High levels of fumarate inhibit alpha ketoglutarate-dependent dioxygenases, including the ten–eleven translocation (TET) enzymes, and can lead to global DNA hypermethylation. Here, we report patterns of hypermethylation in FH-mutant cell lines and tumor samples are associated with the silencing of nicotinate phosphoribosyl transferase (NAPRT), a rate-limiting enzyme in the Preiss–Handler pathway of NAD+ biosynthesis, in a subset of HLRCC cases. NAPRT is hypermethylated at a CpG island in the promoter in cell line models and patient samples, resulting in loss of NAPRT expression. We find that FH-deficient RCC models with loss of NAPRT expression, as well as other oncometabolite-producing cancer models that silence NAPRT, are extremely sensitive to nicotinamide phosphoribosyl transferase inhibitors (NAMPTi). NAPRT silencing was also associated with synergistic tumor cell killing with PARP inhibitors and NAMPTis, which was associated with effects on PAR-mediated DNA repair. Overall, our findings indicate that NAPRT silencing can be targeted in oncometabolite-producing cancers and elucidates how oncometabolite-associated hypermethylation can impact diverse cellular processes and lead to therapeutically relevant vulnerabilities in cancer cells.
Implications: NAPRT is a novel biomarker for targeting NAD+ metabolism in FH-deficient HLRCCs with NAMPTis alone and targeting DNA repair processes with the combination of NAMPTis and PARP inhibitors.
This study was highlighted in the October issue.
Journal: Molecular Cancer Therapeutics
FZ-AD005, a Novel DLL3-Targeted Antibody–Drug Conjugate with Topoisomerase I Inhibitor, Shows Potent Antitumor Activity in Preclinical Models
Delta-like ligand 3 (DLL3) is overexpressed in small cell lung cancer (SCLC) and has been considered an attractive target for SCLC therapy. Rovalpituzumab tesirine was the first DLL3-targeted antibody–drug conjugate (ADC) to enter clinical studies. However, serious adverse events limited progress in the treatment of SCLC with rovalpituzumab tesirine. In this study, we developed a novel DLL3-targeted ADC, FZ-AD005, by using DXd with potent cytotoxicity and a relatively better safety profile to maximize the therapeutic index. FZ-AD005 was generated by a novel anti-DLL3 antibody, FZ-A038, and a valine–alanine (Val–Ala) dipeptide linker to conjugate DXd. Moreover, Fc-silencing technology was introduced in FZ-AD005 to avoid off-target toxicity mediated by FcγRs and showed negligible Fc-mediated effector functions in vitro. In preclinical evaluation, FZ-AD005 exhibited DLL3-specific binding and demonstrated efficient internalization, bystander killing, and excellent in vivo antitumor activities in cell line–derived xenograft and patient-derived xenograft models. FZ-AD005 was stable in circulation with acceptable pharmacokinetic profiles in cynomolgus monkeys. FZ-AD005 was well tolerated in rats and monkeys. The safety profile of FZ-AD005 was favorable, and the highest nonseverely toxic dose was 30 mg/kg in cynomolgus monkeys. In conclusion, FZ-AD005 has the potential to be a superior DLL3-targeted ADC with a wide therapeutic window and is expected to provide clinical benefits for the treatment of patients with SCLC.
This study was highlighted and featured on the cover of the October issue.
Journal: Cancer Research Communications
Synergistic Effects of PARP Inhibition and Cholesterol Biosynthesis Pathway Modulation
An in-depth multiomic molecular characterization of PARP inhibitors revealed a distinct poly-pharmacology of niraparib (Zejula) mediated by its interaction with lanosterol synthase (LSS), which is not observed with other PARP inhibitors. Niraparib, in a similar way to the LSS inhibitor Ro-48-8071, induced activation of the 24,25-epoxysterol shunt pathway, which is a regulatory signaling branch of the cholesterol biosynthesis pathway. Interestingly, the combination of an LSS inhibitor with a PARP inhibitor that does not bind to LSS, such as olaparib, had an additive effect on killing cancer cells to levels comparable with niraparib as a single agent. In addition, the combination of PARP inhibitors and statins, inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an enzyme catalyzing the rate-limiting step in the mevalonate pathway, had a synergistic effect on tumor cell killing in cell lines and patient-derived ovarian tumor organoids. These observations suggest that concomitant inhibition of the cholesterol biosynthesis pathway and PARP activity might result in stronger efficacy of these inhibitors against tumor types highly dependent on cholesterol metabolism.
Significance: The presented data indicate, to our knowledge, for the first time, the potential benefit of concomitant modulation of cholesterol biosynthesis pathway and PARP inhibition and highlight the need for further investigation to assess its translational relevance.


