In 2024, the American Association for Cancer Research (AACR) released the 14th edition of the annual AACR Cancer Progress Report and the third edition of the biennial AACR Cancer Disparities Progress Report that highlight the remarkable progress made in understanding cancer’s complexities, improving early detection and treatment options, and addressing inequities in patient care. Together, these reports complement AACR’s strong commitment to advancing innovative cancer research and ensuring a future in which every individual has equitable and affordable access to life-saving interventions.
The Current State of Cancer
The steady decline in the cancer death rate continues to be a source of immense hope among patients, researchers, and public health experts. In the United States, overall cancer death rates have fallen by 33% between 1991 and 2021, saving more than 4.1 million lives. This progress is driven by decades of groundbreaking research and public health initiatives, which have paved the way for improved detection, more accurate diagnoses, and highly effective treatments.
Despite these advances, cancer remains one of the most pressing public health challenges, both in the United States and globally. In fact, the burden of cancer is projected to rise in the coming decades, and so will its economic impact, placing strain on patients, caregivers, families, and health care systems. Now more than ever, increased federal funding for research is crucial to maintain the pace of progress and sustain public health interventions that ensure continued strides against cancer.
A Spotlight on Young Patients
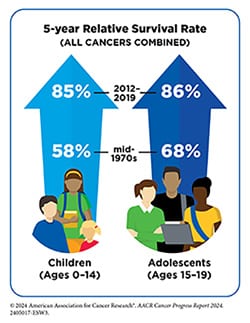
The AACR Cancer Progress Report 2024 included a spotlight on the state of cancer in children, adolescents, and young adults (AYA), which highlighted age-specific cancer risks, research-driven advances in treatment options, and the unique challenges faced by childhood and AYA cancer survivors. The five-year relative survival rate for all cancers combined in these age groups now exceeds 85%, reflecting the power of scientific innovation and improved care.
Scientific breakthroughs have deepened the understanding of how childhood and AYA cancers differ from those in older adults, enabling the development of personalized treatment strategies. These therapies not only target cancer with greater precision but also aim to reduce treatment-related adverse effects, improving both survival rates and quality of life.
Advances in Cancer Care: Precision Medicine at the Forefront of Progress
In the past year, significant progress has been made in cancer therapeutics, with major advances in molecularly targeted therapeutics and immunotherapeutics. Between July 2023 and June 2024, the U.S. Food and Drug Administration (FDA) approved 15 new anticancer therapies, as well as a novel imaging agent for breast cancer surgery and two minimally invasive tests for inherited cancer risk and early detection. Additionally, the use of 15 previously approved therapies was expanded to treat new cancer types. These approvals are covered in detail in the AACR Cancer Progress Report 2024, including:
- Lifileucel – the first tumor-infiltrating lymphocyte (TIL) immunotherapy to be approved by the FDA, and the first cellular therapy to be approved for a solid tumor. This approval will help a high unmet medical need for patients with unresectable or metastatic melanoma, such as Jennifer Ficko, whose cancer had progressed after treatment with other therapies.
- Tarlatamab-dlle – the second bispecific T-cell engager (BiTE) immunotherapy approved by the FDA to treat a solid tumor. Patients with extensive-stage small cell lung cancer (ES-SCLC), a fast-growing lung cancer that has spread to other parts of the body, can now be treated with this therapy after their cancer has progressed on or after platinum-based chemotherapy.
- Adagrasib – a molecularly targeted therapy previously approved for non-small cell lung cancer (NSCLC) that has received an expanded approval in 2024 to be used in combination with cetuximab to treat locally advanced or metastatic colorectal cancer (CRC).
- Clinical trials are now evaluating the potential benefit of this drug in patients with other cancer types, like Humberto M. Guiot, MD, who was diagnosed with late-stage pancreatic cancer. His remarkable response to treatment allowed him to regain much of his life, highlighting the power of clinical trials. Unfortunately, low participation and a lack of ethnic and racial diversity in participants are ongoing challenges in cancer clinical trials. Understanding and eliminating barriers to clinical trials at individual, systemic, and societal levels is crucial to ensure equitable benefits from trials for all patient populations.

As we look to the future, innovative technologies, such as artificial intelligence (AI) and liquid biopsy, hold the promise of revolutionizing early detection and diagnosis and tailoring therapeutic interventions for maximum patient benefit.
Cancer Screening Saves Lives but Gaps Remain
Significant progress has been made in reducing inequities across the cancer continuum. However, disparities persist in various areas, such as cancer prevention, early detection, participation in clinical research, treatment access, and survivorship, uniquely impacting different segments of the U.S. population. Addressing these disparities is particularly important in the context of cancer screening, a critical tool for early detection and prevention that can save millions of lives.
Cancer screening involves checking for cancer or abnormal cells that could develop into cancer, even when there are no noticeable symptoms. By detecting potential issues early—often before they become serious—screening can save lives. This impact is evident in a recent National Cancer Institute study, which reported that nearly 4.75 million deaths were prevented between 1975 and 2020 through timely screening for breast, cervical, colorectal, lung, and prostate cancers.
Cancer screening guidelines, carefully developed by the U.S. Preventive Services Task Force (USPSTF), play a crucial role in prevention and early detection efforts. This independent panel of national experts in prevention and evidence-based medicine evaluates clinical evidence to create recommendations designed to prevent disease and improve health outcomes.
Each recommendation from the USPSTF undergoes a rigorous review process, weighing the benefits and potential harms of a given screening, resulting in a rating that reflects the overall benefit of the screening test. Importantly, these ratings also have real-world implications, as screenings rated favorably are often provided at no cost to patients under the Patient Protection and Affordable Care Act (ACA). By guiding clinical practice, the USPSTF plays a vital role in shaping preventive health care in the United States.
Adhering to cancer screening recommendations is essential for reducing the cancer burden in the United States. However, screening rates are suboptimal for multiple segments of the U.S. population including racial and ethnic minority groups, citizens of sovereign Native Nations, and medically underserved communities.
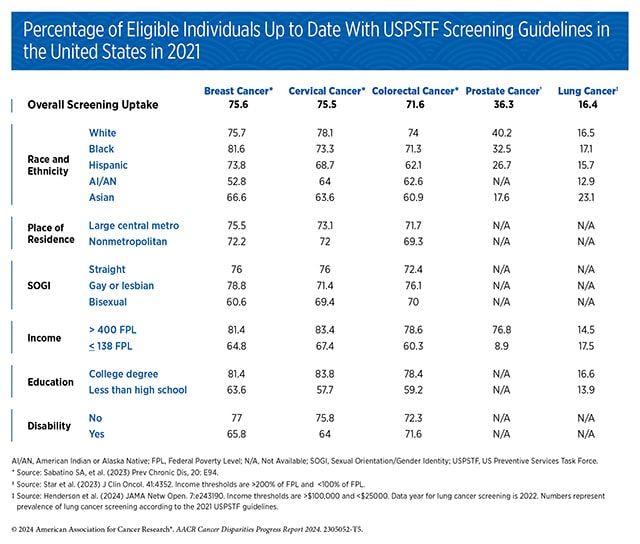
Disparities in cancer screening remain a significant public health challenge, stemming from a complex set of social, structural, and systemic barriers. Many individuals face obstacles such as bias and discrimination within the health care system, leading to mistrust among minoritized populations. Others struggle with limited access to quality health insurance, low health literacy, or breakdowns in communication between patients and providers. Collectively, these factors hinder the utilization and adherence to essential cancer screening services. The AACR Cancer Disparities Progress Report 2024 sheds light on these inequities, focusing on screenings for breast, cervical, prostate, colorectal, and lung cancer. Each of these cancer types have established screening recommendations from the USPSTF, but gaps in access and usage persist.
A Way Forward
Varying levels of adherence to cancer screening and subsequent follow-up care result in disproportionately higher rates of cancers diagnosed at advanced stage and higher cancer mortality among racial and ethnic minority groups, as well as other medically underserved populations. Research has shown that screening rates can be improved using targeted interventions that address the root causes of these disparities—such as, structural racism, discrimination, and other social inequities.
For example:
- U.S. states with expanded Medicaid eligibility have a 16% higher rate of colorectal cancer screening among low-income individuals compared to states without expansion under the ACA.
- The Iowa Get Screened: Colorectal Cancer Program, which helps people with low income get screened, offered patients payment for gas if they had to travel to the clinic to drop off their fecal immunochemical test kit, increasing screening rates from about 33% in 2015 to 57% in 2020
- Bilingual community health workers promoted HPV self-sampling tests among low-income Asian women with limited English proficiency by giving presentations, developing educational materials, and providing follow-up support. As a result, HPV knowledge among the 156 participants doubled, and their comfort with using the self-sampling test increased significantly.
Sustaining the Momentum of Scientific Discovery
Together, the AACR Cancer Progress Report 2024 and AACR Cancer Disparities Progress Report 2024 showcase how medical research continues to extend and improve lives, and emphasize the need for continued research and collaborations to ensure that research-driven advances benefit all communities.
Progress across the basic, translational, clinical, and population research that fuels innovations cannot be made without robust and consistent support. After eight consecutive years of increased federal funding for medical research, Congress reduced National Institutes of Health (NIH) funding in FY 2024, jeopardizing future advancements.
Continued progress in cancer care is not inevitable. Rather, it is the result of bold vision, tireless efforts, and steadfast support from public and private sources, including Congress and philanthropic organizations. Sustained and predictable investment in cancer research is the foundation of every breakthrough, and the driver of innovations and interventions that reduce cancer risk, improve early detection, advance effective treatments, and promote health equity.


