When the U.S. Food and Drug Administration (FDA) announced in November 2023 that it was investigating a link between receiving CAR T therapy and developing secondary cancers later on, it spurred uncertainty for patients and researchers alike.
As many of the more than 30,000 patients who have received the life-saving treatment face new questions about their risks, researchers have used the FDA announcement as a springboard for studies of CAR T outcomes to shed light on the subject. Preliminary findings from a systematic review and meta-analysis recently published in the American Association for Cancer Research (AACR) journal Clinical Cancer Research may suggest hope for patients feeling anxious about their future.
What Is the Problem?
A major breakthrough in cancer treatments happened when the first CAR T-cell therapy—a type of therapy where patients’ own T cells are isolated, genetically engineered to attack cancer, and infused back into the patient—reached the market in 2017. A total of six types of CAR T therapy have been approved by the FDA to treat certain lymphomas, leukemias, and/or multiple myelomas:
- tisagenlecleucel (Kymriah, first approved in 2017);
- axicabtagene ciloleucel (Yescarta, first approved in 2017);
- brexucabtagene autoleucel (Tecartus, first approved in 2020);
- lisocabtagene maraleucel (Breyanzi, first approved in 2021);
- idecabtagene vicleucel (Abecma, first approved in 2021); and
- ciltacabtagene autoleucel (Carvykti, first approved in 2022).
All six treatments consist of T cells that are genetically engineered to target proteins on the surface of B cells. When the engineered T cells are infused back into the patient, they attack the B cells, including the malignant B cells that make up multiple myeloma or some lymphomas and leukemias.
Genetic engineering of cells that are returned to a patient’s body is a relatively new process and, at the mechanistic level, is not without risks. When researchers introduce new genes to the T cells, they do not always have control over where the genes are inserted into the genome. There is a small but tangible chance a new gene could jump into the middle of a tumor suppressor gene, weakening the T cell’s natural defenses against becoming cancerous itself.
For this reason, the clinical trials that led to the approval of existing CAR T therapies carefully monitored patients for the development of T-cell cancers. Once approved, the FDA required a class warning label on the therapies’ prescribing information alerting to the possibility of secondary malignancies, and they required drug developers to conduct postmarketing studies with at least 15 years of follow-up.
On November 28, 2023, the FDA announced that they were investigating reports of secondary T-cell cancers arising in patients who had been previously treated with CAR T therapy. The reports were derived from clinical trial data and postmarketing adverse event databases such as the FDA Adverse Event Reporting System (FAERS) and included cancers that arose specifically from T cells containing the inserted CAR gene. Reports were not limited to a single CAR T product, prompting a class-wide evaluation of risk.
This led to a decision in January 2024 to require a boxed warning—a special alert that warns of potentially lethal side effects—in the safety labeling of each CAR T therapy that communicates the risk of secondary T-cell malignancies. The labeling updates were completed by April 2024, and the FDA issued another announcement explaining their decision.
Since the FDA’s initial communication, several research groups have launched their own investigations to help contextualize the risk for patients who may be anxious about their odds of developing another cancer. A study of patients treated with CAR T therapies at the University of Pennsylvania found that 16 out of 449 patients (3.6%) developed a secondary cancer after a median follow-up of 10 months; only one case was a T-cell lymphoma. Another study of 724 CAR T patients treated at Stanford found only one T-cell lymphoma which did not appear to contain the edited CAR gene.
A third study examined the incidence of secondary cancers within the FAERS database and found 19 T-cell malignancies among 12,394 total adverse events reported after CAR T. The database included a markedly high rate of T-cell cancers and myeloid cancers, which prompted the researchers to conduct a disproportionality analysis. They concluded that the data may be subject to a reporting bias, in which patients are more likely to report severe side effects, such as cancer, to the database, which could potentially skew risk estimates.
New Data Provides Perspective and Offers Hope
While individual studies suggested that the risk of secondary T-cell cancers after CAR T therapy was relatively low, each had its own limitations regarding cohort size, specific treatments used, and duration of follow-up. These factors limited the extent to which researchers could tease out what patient- and treatment-related characteristics contributed to an increased risk.

“Patients are reading this in the news and, appropriately, asking questions to providers,” said Kai Rejeski, MD, a visiting investigator and research fellow in the Adult Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center, in a press release. “We need to understand the potential risks, but at the same time, we need to interpret the data cautiously and contextualize it for our patients.”
To amass enough data for more granular analyses, Rejeski and colleagues combined the data from several studies, performing a systematic review and meta-analysis. They searched the available literature for studies in which adult patients with B-cell lymphoma or multiple myeloma received a currently approved CAR T therapy and were monitored for secondary cancers throughout the full follow-up period. Their final selection included 18 clinical trials and seven real-world studies that followed patients for a median of 21.7 months.
Across all studies, a total of 326 secondary cancers occurred in 5,517 patients treated with CAR T; the overall rate of secondary malignancies was 5.8%, which Rejeski noted was similar to rates observed in other studies. Most of these (37%) were hematologic malignancies, but only five cases were T-cell cancers, a rate of 0.09% across the full study population. Three of these cases were tested for the engineered CAR gene, and one tested positive.
Rate of secondary cancers after CAR T therapy similar to rate after other treatments
Notably, Rejeski and colleagues also analyzed the rate of secondary cancers in four studies that included a control group in which patients were treated with standard-of-care therapy.
In a total of 1,253 patients across these studies, the rate of secondary cancer was 5% among patients treated with CAR T and 4.9% among patients treated with the standard of care, suggesting that the risk of secondary cancer was no greater among patients treated with CAR T than those treated with other therapies.
The researchers assessed whether the type of CAR T therapy or the patient’s diagnosis correlated with the rate of secondary cancers, but neither appeared to have an effect. Two characteristics, however, did significantly increase the odds of developing secondary cancers. One was more lines of prior therapy; studies in which patients had received a median of three or more prior therapies reported a secondary cancer rate of 8.7% while those in which patients received fewer than three prior therapies reported a rate of 5.7%.
CAR T therapy experts and University of Pennsylvania Perelman School of Medicine researchers Marco Ruella, MD, an assistant professor of medicine, and Carl H. June, MD, FAACR, the Richard W. Vague Professor in Immunotherapy and director of both the Center for Cellular Immunotherapies and the Parker Institute for Cancer Immunotherapy, reflected on this in a commentary in the AACR journal Blood Cancer Discovery, where they discussed other cellular mechanisms that could also confound the data on secondary malignancies after receiving CAR T therapy. Most patients receiving CAR T have already received other lines of therapy—including chemotherapy, radiation, and stem-cell transplants—that are known to increase the risk of secondary cancers.
Even in cases where the CAR gene was found in malignant cells, a cancerous clone may have existed prior to engineering, and the contributions of CAR gene insertion in the process of malignant transformation may have been negligible, Ruella and June note.
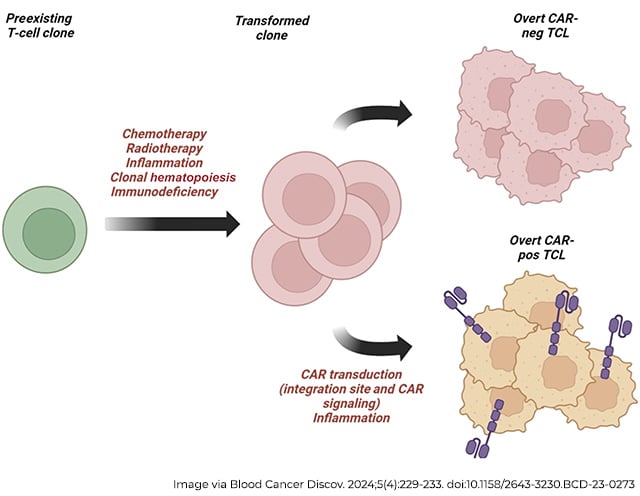
“It is important to recall that associations do not imply causality,” Ruella and June wrote in their commentary. “Deep analyses are needed to fully understand the relative contribution of the CAR transduction versus germline mutations and previous chemotherapy.”
Rajeski and colleagues also found in their analysis that the other factor that significantly impacted the rate of secondary cancers was follow-up time; studies with a follow-up time longer than the median reported a secondary cancer rate of 8.5% as compared to 4.2% among studies with a follow-up time shorter than the median. Rejeski suggested that these data may reflect a survivorship bias, meaning more patients may be developing secondary cancers simply because they’re living long enough to do so.
“You have to be alive to develop secondary malignancies,” he said. “CAR T therapy is the first treatment in more than 20 years to show an overall survival benefit compared to the standard of care in refractory large B-cell lymphoma.”
“These data do not suggest that there is an increased risk of second primary malignancies relative to other standard-of-care therapies,” he continued. “I would strongly caution against withholding this therapy because of the miniscule risk of developing T-cell malignancies.”
Rejeski emphasized that more work will be necessary to better understand the respective contributions of CAR T therapy versus other confounding factors when assessing the risk of secondary malignancies, and that the first step is to ensure the robust collection of data. “We still need to be vigilant in understanding this adverse event, and that means we need to have clear reporting standards,” Rejeski said in a press release.


